1. Valdés, L. J., III; Díaz, J. L.; Paul, A. G. J. Ethnopharmacol.
1983, 27, 287-312.
2. Valdés, L. J., III; Butler, W. M.; Hatfield, G. M.; Paul, A. G.; Koreeda,
M. J. Org. Chem. 1984, 49, 4716-4720.
3. Valdés, L. J., III. J. Nat. Prod. 1986, 49,
171.
4. Valdés, L. J., III; Hatfield, G. M.; Koreeda, M.; Paul, A. G. Econ.
Bot. 1987, 41, 283-291.
5. Valdés, L. J., III. J. Psychoactive Drugs 1994, 26,
277-283.
6. Koreeda, M.; Brown, L.; Valdes, L. J., III. Chem Lett. 1990,
2015-2018.
7. Ortega, A.; Blount, J. F.; Marchand, P. S. J. Chem. Soc.,
Perkin Trans. 1 1982, 2505-2508.
8. Harada, N.; Nakanishi, K. Circular Dichroic Spectroscopy-Exciton
Coupling in Organic Stereochemistry; University Science Books: Mill
Valley, CA, 1983.
9. Valdés, L. J., III. Ph.D. Dissertation, University of Michigan, Ann Arbor,
Michigan, 1983. See also: Brimblecombe, R. W.; Green, A. L. Nature
(London) 1962, 45, 983.
10. Siebert, D. J. J. Ethnopharmacol. 1994, 43,
53-56.
11. Schultes, R. E.; Hofmann, A. The Botany and Chemistry of
Hallucinogens; Charles, C., Ed.; Thomas Publisher: Springfield, IL,
1980.
12. A 10- m Radial Pak Microporasil
silica gel column (10 cm × 8 mm id) eluted with an isocratic solvent mixture of
10% acetonitrile, 30% methyl-tert-butyl ether, and 60% hexanes with a
flow rate of 1.5 mL/min.
m Radial Pak Microporasil
silica gel column (10 cm × 8 mm id) eluted with an isocratic solvent mixture of
10% acetonitrile, 30% methyl-tert-butyl ether, and 60% hexanes with a
flow rate of 1.5 mL/min.
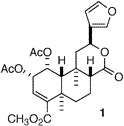
13. Salvinorin C (1): IR (KBr) 3150, 2950, 2920, 2850, 1755, 1735,
1715, 1635, 1430, 1370, 1310, 1225, 1140, 1070, 1035, 955, 905, 870, 785, 765
cm-1; HRMS (EI) m/z calcd for
C25H30O9 474.1890, found 474.1865.
14. Data for the 8-epimer of diol 4: mp 234-235  C (EtOH); [
C (EtOH); [ ]22D +8.8
(c 0.24, MeOH); 1H NMR (400 MHz, acetone-d6)
]22D +8.8
(c 0.24, MeOH); 1H NMR (400 MHz, acetone-d6)
 0.97 (d, 1H, J = 1.1
Hz), 1.33 (s, 3H), 1.43 (ddd, 1H, J = 13.7, 4.5, 3.9 Hz), 1.60 (ddd, 1H,
J = 13.7, 13.6, 5.0 Hz), 1.58-1.68 (m, 1H), 1.70 (s, 3H), 1.82 (dd, 1H,
J = 12.1, 11.6 Hz), 1.91 (dddd, 1H, J = 13.6, 13.3, 4.7, 3.9 Hz),
2.03 (dddd, 1H, J = 13.3, 5.0, 4.5, 1.9 Hz), 2.14 (dd, 1H, J =
11.6, 1.9 Hz), 2.16 (dd, 1H, J = 9.7, 1.9 Hz), 2.17 (ddd, 1H, J =
12.0, 11.5, 9.7 Hz), 2.60 (dd, 1H, J = 4.7, 1.9 Hz), 2.86 (s, 1H, 1-OH),
2.89 (s, 1H, 2-OH), 3.60 (ddd, 1H, J = 11.5, 4.5, 2.3 Hz), 3.62 (s, 3H),
4.09 (dd, 1H, J = 2.3, 1.1 Hz), 5.49 (ddd, 1H, J = 12.1, 1.9, 1.2
Hz), 6.58 (dd, 1H, J = 1.8, 0.7 Hz), 7.57 (dd, 1H, J = 1.8, 1.7
Hz), 7.66 (ddd, 1H, J = 1.7, 1.2, 0.7 Hz); 13C NMR (75 MHz,
acetone-d6)
0.97 (d, 1H, J = 1.1
Hz), 1.33 (s, 3H), 1.43 (ddd, 1H, J = 13.7, 4.5, 3.9 Hz), 1.60 (ddd, 1H,
J = 13.7, 13.6, 5.0 Hz), 1.58-1.68 (m, 1H), 1.70 (s, 3H), 1.82 (dd, 1H,
J = 12.1, 11.6 Hz), 1.91 (dddd, 1H, J = 13.6, 13.3, 4.7, 3.9 Hz),
2.03 (dddd, 1H, J = 13.3, 5.0, 4.5, 1.9 Hz), 2.14 (dd, 1H, J =
11.6, 1.9 Hz), 2.16 (dd, 1H, J = 9.7, 1.9 Hz), 2.17 (ddd, 1H, J =
12.0, 11.5, 9.7 Hz), 2.60 (dd, 1H, J = 4.7, 1.9 Hz), 2.86 (s, 1H, 1-OH),
2.89 (s, 1H, 2-OH), 3.60 (ddd, 1H, J = 11.5, 4.5, 2.3 Hz), 3.62 (s, 3H),
4.09 (dd, 1H, J = 2.3, 1.1 Hz), 5.49 (ddd, 1H, J = 12.1, 1.9, 1.2
Hz), 6.58 (dd, 1H, J = 1.8, 0.7 Hz), 7.57 (dd, 1H, J = 1.8, 1.7
Hz), 7.66 (ddd, 1H, J = 1.7, 1.2, 0.7 Hz); 13C NMR (75 MHz,
acetone-d6)  17.36 (q), 18.91 (t), 26.59 (q), 29.77 (t), 37.43 (s), 37.51 (s), 37.85 (t),
46.85 (d), 49.67 (t), 51.15 (q), 55.38 (d), 55.48 (d), 70.08 (d), 70.21 (d),
71.93 (d), 109.66 (d), 125.90 (s), 140.80 (s), 144.38 (d), 173.75 (s), 174.31
(s). Anal. Calcd for C21H28O7: C, 64.60; H,
7.23. Found: C, 64.14; H, 7.18.
17.36 (q), 18.91 (t), 26.59 (q), 29.77 (t), 37.43 (s), 37.51 (s), 37.85 (t),
46.85 (d), 49.67 (t), 51.15 (q), 55.38 (d), 55.48 (d), 70.08 (d), 70.21 (d),
71.93 (d), 109.66 (d), 125.90 (s), 140.80 (s), 144.38 (d), 173.75 (s), 174.31
(s). Anal. Calcd for C21H28O7: C, 64.60; H,
7.23. Found: C, 64.14; H, 7.18.
15. Valdes, L. J., III.; Koreeda, M. J. Org. Chem. 1991,
56, 844-846.
16. Data for 5: mp 206-209  C
(hexanes/EtOAc); [
C
(hexanes/EtOAc); [ ]22D +7.1 (c 0.70, CHCl3);
1H NMR (400 MHz, CDCl3)
]22D +7.1 (c 0.70, CHCl3);
1H NMR (400 MHz, CDCl3)  1.16 (s, 3H), 1.36 (s, 3H), 1.42
(ddd, 1H, J = 13.9, 13.0, 3.6 Hz), 1.47 (d, 1H, J = 1.7 Hz), 1.60
(dddd, 1H, J = 14.1, 13.9, 12.1, 2.9 Hz), 1.62 (d, 1H, J = 1.7
Hz), 1.72 (ddd, 1H, J = 13.0, 3.5, 2.9 Hz), 1.73 (dddd, 1H, J =
13.0, 4.9, 2.8, 1.0 Hz), 1.90 (dd, 1H, J = 13.2, 11.7 Hz), 2.00 (dddd,
1H, J = 14.1, 3.6, 3.5, 3.2 Hz), 2.07 (s, 3H), 2.11 (ddd, 1H, J =
13.2, 13.0, 12.1 Hz), 2.32 (dd, 1H, J = 13.2, 2.8 Hz), 2.35 (dd, 1H,
J = 12.1, 3.2 Hz), 2.48 (dd, 1H, J = 13.2, 5.4 Hz), 3.64 (s, 1H,
OH), 3.65 (s, 3H), 3.68 (ddd, 1H, J = 12.1, 4.9, 1.7 Hz), 5.54 (dd, 1H,
J = 11.7, 5.4 Hz), 5.60 (ddd, 1H, J = 1.7, 1.7, 1.0 Hz), 6.59 (dd,
1H, J = 1.8, 0.8 Hz), 7.57 (dd, 1H, J = 1.8, 1.5 Hz), 7.68 (ddd,
1H, J = 1.5, 0.8, 0.8 Hz). Anal. Calcd for
C23H30O8: C, 63.58; H, 6.96. Found: C, 63.42;
H, 7.00.
1.16 (s, 3H), 1.36 (s, 3H), 1.42
(ddd, 1H, J = 13.9, 13.0, 3.6 Hz), 1.47 (d, 1H, J = 1.7 Hz), 1.60
(dddd, 1H, J = 14.1, 13.9, 12.1, 2.9 Hz), 1.62 (d, 1H, J = 1.7
Hz), 1.72 (ddd, 1H, J = 13.0, 3.5, 2.9 Hz), 1.73 (dddd, 1H, J =
13.0, 4.9, 2.8, 1.0 Hz), 1.90 (dd, 1H, J = 13.2, 11.7 Hz), 2.00 (dddd,
1H, J = 14.1, 3.6, 3.5, 3.2 Hz), 2.07 (s, 3H), 2.11 (ddd, 1H, J =
13.2, 13.0, 12.1 Hz), 2.32 (dd, 1H, J = 13.2, 2.8 Hz), 2.35 (dd, 1H,
J = 12.1, 3.2 Hz), 2.48 (dd, 1H, J = 13.2, 5.4 Hz), 3.64 (s, 1H,
OH), 3.65 (s, 3H), 3.68 (ddd, 1H, J = 12.1, 4.9, 1.7 Hz), 5.54 (dd, 1H,
J = 11.7, 5.4 Hz), 5.60 (ddd, 1H, J = 1.7, 1.7, 1.0 Hz), 6.59 (dd,
1H, J = 1.8, 0.8 Hz), 7.57 (dd, 1H, J = 1.8, 1.5 Hz), 7.68 (ddd,
1H, J = 1.5, 0.8, 0.8 Hz). Anal. Calcd for
C23H30O8: C, 63.58; H, 6.96. Found: C, 63.42;
H, 7.00.
17. King, J. F.; Allbutt, A. D. Can. J. Chem. 1970, 48,
1754-1769.
18. Data for 7: mp 211-214  C
(hexanes/EtOAc); [
C
(hexanes/EtOAc); [ ]22D -7.5 (c 0.81, CHCl3);
1H NMR (400 MHz, acetone-d6)
]22D -7.5 (c 0.81, CHCl3);
1H NMR (400 MHz, acetone-d6)  1.16 (s, 3H), 1.38 (s, 3H), 1.50
(dddd, 1H, J = 14.0, 12.8, 12.2, 2.6 Hz), 1.62 (d, 1H, J = 1.7
Hz), 1.63 (ddd, 1H, J = 13.0, 12.8, 3.2 Hz), 1.76 (dddd, 1H, J =
12.9, 4.8, 2.8, 1.2 Hz), 1.78 (ddd, 1H, J = 13.0, 3.2, 3.0 Hz), 1.90 (s,
3H), 1.94 (dd, 1H, J = 13.2, 11.7 Hz), 2.02 (dddd, 1H, J = 14.0,
3.3, 3.2, 3.0 Hz), 2.14 (s, 3H), 2.23 (ddd, 1H, J = 13.2, 12.9, 12.4 Hz),
2.32 (dd, 1H, J = 13.2, 5.5 Hz), 2.40 (dd, 1H, J = 12.2, 3.3 Hz),
2.45 (dd, 1H, J = 13.2, 2.8 Hz), 3.67 (s, 3H), 4.81 (ddd, 1H, J =
12.4, 4.8, 3.4 Hz), 5.56 (dd, 1H, J = 11.7, 5.5 Hz), 5.68 (ddd, 1H,
J = 3.4, 1.7, 1.2 Hz), 6.58 (dd, 1H, J = 1.8, 0.8 Hz), 7.56 (dd,
1H, J = 1.8, 1.5 Hz), 7.66 (ddd, 1H, J = 1.5, 0.8, 0.8 Hz). Anal.
Calcd for C25H32O9: C, 63.01; H, 6.77. Found:
C, 62.87; H, 6.71.
1.16 (s, 3H), 1.38 (s, 3H), 1.50
(dddd, 1H, J = 14.0, 12.8, 12.2, 2.6 Hz), 1.62 (d, 1H, J = 1.7
Hz), 1.63 (ddd, 1H, J = 13.0, 12.8, 3.2 Hz), 1.76 (dddd, 1H, J =
12.9, 4.8, 2.8, 1.2 Hz), 1.78 (ddd, 1H, J = 13.0, 3.2, 3.0 Hz), 1.90 (s,
3H), 1.94 (dd, 1H, J = 13.2, 11.7 Hz), 2.02 (dddd, 1H, J = 14.0,
3.3, 3.2, 3.0 Hz), 2.14 (s, 3H), 2.23 (ddd, 1H, J = 13.2, 12.9, 12.4 Hz),
2.32 (dd, 1H, J = 13.2, 5.5 Hz), 2.40 (dd, 1H, J = 12.2, 3.3 Hz),
2.45 (dd, 1H, J = 13.2, 2.8 Hz), 3.67 (s, 3H), 4.81 (ddd, 1H, J =
12.4, 4.8, 3.4 Hz), 5.56 (dd, 1H, J = 11.7, 5.5 Hz), 5.68 (ddd, 1H,
J = 3.4, 1.7, 1.2 Hz), 6.58 (dd, 1H, J = 1.8, 0.8 Hz), 7.56 (dd,
1H, J = 1.8, 1.5 Hz), 7.66 (ddd, 1H, J = 1.5, 0.8, 0.8 Hz). Anal.
Calcd for C25H32O9: C, 63.01; H, 6.77. Found:
C, 62.87; H, 6.71.
![]()
![]()
![]() and
and ![]()
![]()